Clinical Trials
As part of our mission, Dup15q Alliance seeks to unite families, researchers, and professionals; and promote research, awareness, and empower individuals with dup15q syndrome by advancing breakthrough research and life-changing therapeutic treatments. Dup15q Alliance formally endorses and funds research and collaborates with researchers interested in research on chromosome 15q duplications by disseminating research information and promoting opportunities for Dup15q Alliance families to participate in research studies and clinical trial opportunities.
Choosing to participate in a clinical trial is an important personal decision. It is often helpful to talk to a physician, family members, or friends about deciding to participate in a clinical trial.
Sign up for our NEW Research and Clinical Trial Text Alerts! You can unsubscribe from text alerts at any time.
Use this link below or Text “Alerts” to (847) 744-8904 to sign up!
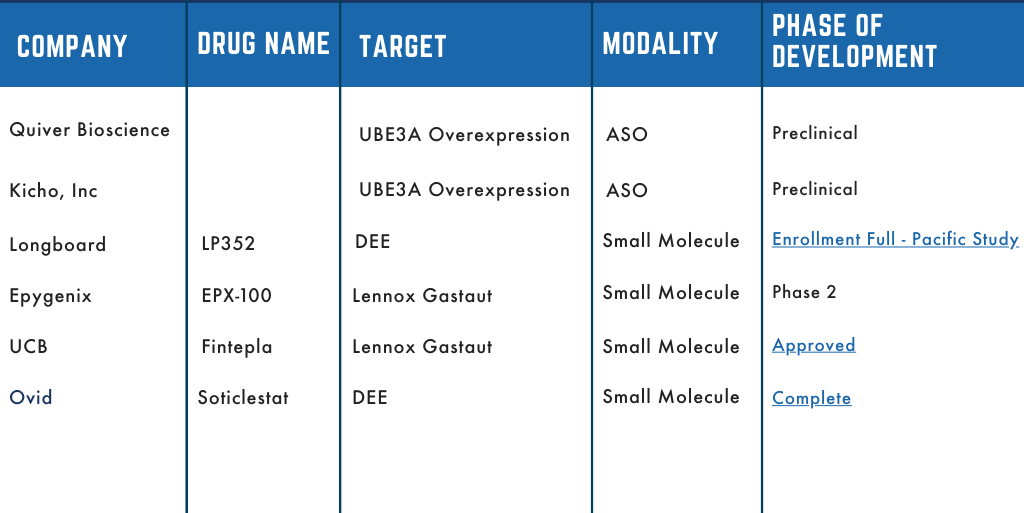
Dup15q Therapeutic Pipeline
The Dup15q Alliance has been at the forefront of collaborative efforts with stakeholders in both the academic and pharmaceutical spaces. We’ve aimed to accelerate research efforts to develop therapeutic interventions for those with Dup15q. Several of those partnerships have moved into clinical development and we are thrilled to share our pipeline. The pipeline outlines which company is developing the therapeutic, the name of the asset/drug, what the drug is targeting and the stage of development.
Stay tuned as these drugs move through development, the pipeline will be updated to reflect their stage of progression.
What is a Clinical Trial?
Clinical trials are research studies involving human participants that are done to test new treatments and medications. A clinical trial can only be conducted if all possible precautions have been taken to protect patient safety and when there is reason to believe the new therapy will improve care, quality of life, and other things. All clinical trials are run in accordance with the Principles of Good Clinical Practice (GCP), which are overseen by independent ethics committees (IEC and IRB).
What is the Treatment?
New medications/devices are first tested as a treatment through extensive laboratory research. If the initial laboratory research is successful, the researchers send the data to the Food and Drug Administration (FDA) for approval to continue research in clinical trials.
Already existing medications/devices that are used for other diagnoses are often used in clinical trials as a potential new treatment option.
Why participate in a clinical trial?
Clinical trials are required for any new therapy prior to FDA approval. Major improvements in health care for rare disorders like dup15q syndrome would be impossible without volunteer participants. Participants in clinical trials can play a more active role in their own health care, gain access to new research treatments before they are widely available, and help others by contributing to medical research. The more people who participate in clinical trials, the faster drugs, treatments, and lifestyle changes can be tested and the quicker people can get better care.
How does participating in a clinical trial help the Dup15q Community?
We need to have a certain number of patients in the Dup15q Community enrolled in drug trials to ensure it is available to all those affected by Dup15q Syndrome when it goes to market.
When new treatment/drugs are being developed to address disorders or symptoms of disorders the developers choose patient populations to focus on the efficacy and effectiveness in that particular disorder so when the drug goes to the next steps of approval that disorder and the data of its effectiveness goes into the indication (that disorders are listed a treatment area for the drug).
Phases of a Clinical Trial
Each phase is designed to answer certain questions while keeping the people taking part as safe as possible. Results from these phases show if the new drug or treatment is reasonably safe and effective.
Phase 1
Is It Safe for Humans?
A Phase 1 clinical trial tests a potential new treatment in people for the first time, sometimes called “first-in-human studies.” The primary goal of this testing is to determine if the treatment is safe for humans.
- Conducted with healthy volunteers and emphasize safety. May enroll patients who have the disease.
- Find out what the therapy’s most frequent and serious adverse events are.
- Understand how the therapy is metabolized and excreted.
- Determine safe levels to be administered
Phase 2
Is It Safe and Effective for the Target Disease?
The goal of a Phase 2 trial is to find out if the new therapy safely and effectively treats its target disease. Data is collected for a few months to 2 years.
- Requires more participants, and these participants must have the disease that the new therapy is intended to treat.
- Continued evaluation of safety and monitoring of short-term adverse events.
- Determine whether the therapy works as expected in people who have a certain disease or condition.
- May compare participants receiving the therapy to similar participants receiving an inactive substance (called a placebo).
Phase 3
Can Phase 2 Findings Be Confirmed?
The goal of Phase 3 trials is to confirm or disprove the findings made in Phase 2. Data is collected for 1 to 4 years.
- Monitor short-term and longer-term side effects
- Determine if the therapy continues to work as expected in a larger group of patients.
- May include gathering data to determine the most effective dosage.
Phase 4
Does It Perform as Expected in the “Real World”?
The goal of these Phase IV clinical trials is to assess the new treatment’s performance in the “real world.” Data is collected for many years. This is important because prior to its approval, the new treatment was only examined in a study setting.
- Occur after FDA has approved a therapy for marketing.
- Gather additional information about a therapy’s safety, efficacy, and optimal use.
- Enroll a large patient population, with eligibility requirements determined by the FDA-approved indications.
Clinical Trial FAQ
The U.S. National Library of Medicine maintains a website, www.clinicaltrials.gov. This website provides a brief overview of clinical research, information for potential clinical study participants, and a glossary of common words used on ClinicalTrials.gov.
Who can participate in a clinical trial?
Each clinical trial has a protocol that describes who is eligible to take part in the research. Each study must include only people who meet the requirements for that study. These are the study’s eligibility criteria.
Eligibility criteria are different for each trial. These criteria are based on the goals of the study and are used to identify appropriate participants, keep them safe, and help ensure that researchers can find the new information they need. They include such factors as age, the type, and stage of a disease, previous treatment history, and other medical conditions.
Why participate in a clinical trial?
Clinical trials are required for any new therapy prior to FDA approval. Major improvements in health care for rare disorders like dup15q syndrome would be impossible without volunteer participants.
Participants in clinical trials can play a more active role in their own health care, gain access to new research treatments before they are widely available, and help others by contributing to medical research.
The more people who participate in clinical trials, the faster drugs, treatments, and lifestyle changes can be tested and the quicker people can get better care. Clinical trials have many potential medical benefits, including:
- Identifying effective treatments for future generations
- Accelerating research for new and innovative treatments
- Lowering the cost of therapy through a better understanding of which patients may benefit from a specific type of therapy
- Giving researchers more complete data
What are the risks?
A clinical trial can only be conducted if all possible precautions have been taken to protect patient safety, and an investigational drug can only be tested if there is a real possibility of therapeutic benefit. All clinical trials are run in accordance with the Principles of Good Clinical Practice (GCP), which are overseen by independent ethics committees (IEC and IRB).
- The physician running a clinical trial will ensure that every patient invited to participate is provided with information about potential adverse events and has time to consider the risks.
- Patients are given time to reflect before they decide whether to enter a trial. If they do decide to participate, they will be walked through the risks and asked to confirm that they are aware of the study procedures, risks, and constraints.
- Patients who participate in clinical trials are given the highest degree of protection throughout the study through biological, medical, and clinical supervision.
- Patients may decide at any time to withdraw from the study.
How does participation help others in the dup15q community?
We need to have a certain number of patients in the Dup15q Community enrolled in drug trials to ensure it is available to all those affected by Dup15q Syndrome when it goes to market.
When new treatment/drugs are being developed to address disorders or symptoms of disorders the developers choose patient populations to focus on the efficacy and effectiveness in that particular disorder so when the drug goes to the next steps of approval that disorder and the data of its effectiveness goes into the indication (that disorders are listed a treatment area for the drug)
What happens during the clinical trial?
Before anyone joins a study in any phase as a participant, you will meet with the researchers conducting the trial to discuss relevant aspects of the study and go over the informed consent form. Specifically, each study will vary in terms of who can participate, how many participants are needed, what tests will be used, how long the study will last, and how the data will be analyzed.
It will also vary in terms of the time, money, and energy required of you and your family if you choose to participate. You should ask as many questions of the researchers conducting the trial to get the information you need to decide whether participation is right for you and your family.
During the clinical trial, your clinical trial study team which includes doctors, nurses, and other healthcare professionals, who will provide your healthcare. They check the health of the participant at the beginning of the trial, gives specific instructions for participating in the trial, will do the tests and exams related to the study, monitors the participant carefully during the trial, and stays in touch after the trial is completed. They will usually share your results and provide other information to your personal doctor(s) if you allow.
The clinical trial study team, which includes doctors, nurses, and other healthcare professionals, checks the health of the participant at the
beginning of the trial, gives specific instructions for participating in the trial, monitors the participant carefully during the trial, and stays in touch after the trial is completed.
Some clinical trials involve more tests and doctor visits than the participant would normally have for an illness or condition. Clinical trial participation is most successful when the study protocol is carefully followed, including frequent contact with the clinical trial team.
Clinical trial study team: A clinical trial team is made up of many different key members. The team may vary depending on several factors including the type and phase of clinical trial and whether the trial involves multiple centers. Key roles include:
-
- Principal Investigator (PI): A PI is the lead researcher or clinician who is conducting the clinical trial. The PI is responsible for:
- Ensuring that an investigation is conducted according to the signed investigator statement, the investigational plan, and applicable regulations.
- Protecting the rights, safety and welfare of the subjects under the investigator’s care.
- Control of the therapy(s) under investigation.
- Clinical Research Coordinator (CRC): A CRC manages and conducts the day-to-day study activities in accordance with the protocol, applicable regulations, and Good Clinical Practice (GCP) and Institutional Review Board (IRB ) requirements.
- Sub Investigator (Sub I): The Sub I is a member of the research team designated and supervised by the PI to perform critical study-related procedures and/or to make important study-related decisions.
- Clinical Research Nurse Coordinator (CRNC): A CRNC may be required for certain protocol-related activities.
- Regulatory Coordinator: The regulatory coordinator prepares and maintains IRB submissions and FDA documents. May also track study progress.
- Principal Investigator (PI): A PI is the lead researcher or clinician who is conducting the clinical trial. The PI is responsible for:
After the data is collected from the study, the clinical trial team analyzes it to identify the key findings. The team uses these findings to determine what to do next with the therapy. The next step could be the next phase of clinical trials if the data look promising, and the regulatory agency agrees. However, if the therapy does not meet set safety and/or efficacy standards, no further investigation will be performed.
What does indication mean and why is it important?
The “indication” for a drug refers to the use of that drug for treating a particular disease/disorder. A drug can have more than one FDA-labeled indication, this means it can be used for multiple medical conditions. The primary role of the indication labeling is to enable health care practitioners to readily identify appropriate therapies for patients by clearly communicating the drug’s approved indication(s).
Off-Label Use
When looking at your treatment options, your healthcare provider may decide to have you use an off-label medication differently than how it is normally prescribed or for what it is indicated for. Off-Label use is defined as using an FDA-approved medication is used in a way that has not been approved by the FDA through a standard clinical trial for safety and efficacy.
Examples of off-label uses can include, but are not limited to:
-
When a medication is used for a condition that it is not approved to treat
-
When a medication is dosed differently than what has been approved
-
Treating children with a medication that has been approved for use in adults only
Off-label uses may eventually get approved by the FDA.
Insurance Coverage
An indication is also important in terms of insurance and medical billing. Some medical insurance policies may not cover “off-label” use of medication. Medicare Part D covers drugs prescribed for off-label use only if the drugs are identified as safe and effective for that use in one of three officially recognized drug compendia. “Compendia” are encyclopedias of drug chemicals, with information on dosage and usage.
There are certainly risks in inferring indication from diagnosis, especially if the prescriber is using medication for an off-label purpose.
What is a protocol?
A protocol is a study plan on which each clinical trial is based. The plan is carefully designed to safeguard the health of the participants as well as
answer specific research questions. A protocol describes what types of people may participate in the trial; the schedule of tests, procedures, medications, and dosages; and the length of the study.
What is a placebo?
A placebo is an inactive pill, liquid, or powder that has no treatment value. In clinical trials, study treatments are often compared with placebos to assess the study treatment’s effectiveness. In some studies, the participants in the control group will receive a placebo instead of an active drug or experimental treatment.
What is a control group?
A control is the standard by which study observations are evaluated. In many clinical trials, one group of study participants will be given an investigational (also called “study”) drug or treatment, while the control group is given either a standard treatment for the illness or a placebo.
Do I get to choose what group I participate in? (Experimental or control)
No, each person who agrees to participate in a clinical trial that compares a study drug or device with a standard treatment or placebo is randomly
assigned (that is, by chance) to one of the groups. In general, the participant and the clinical trial team do not know the group assignment
until after the study is completed.
What difference does it make if I know or the clinical team trial team knows what group I am in?
Knowledge of this information may influence a participant’s or study team’s reporting of how things are going in the study. For example, if the
participant and/or study team knows that the participant is in the study group, an adverse event such as a skin rash might be reported as being
“likely” related to the study drug, instead of “possibly related.” Or the participant might report adverse events more frequently than if he/she was unaware of the group assignment. However, if the participant and/ or study team knows that the participant is in the control/placebo group, the skin rash would be reported as “unrelated” and, more importantly, the participant in this group might unintentionally report worsening of his/her illness or condition, when there has been no change.
What does single blind or double blind study mean?
“Blinding” is a procedure in which one or more persons in the research trial are kept unaware of the treatment assignment(s). Single-blind usually means that the research participant is not told of the treatment assignment. Double-blind usually means that the research participant, investigator, study coordinator/ nurse, study sponsor, and in some cases the data analyst, are kept unaware of the treatment assignment. The purpose of a “blinded” study design is to remove the unintentional bias that can affect the interpretation of the research information that is collected, if the treatment assignment is known. If the participant’s safety requires it, an independent Data Safety Monitoring Board can rapidly tell the investigator which treatment assignment a participant was given; however, this generally requires the participant to withdraw from further participation in that study.
Is an adverse affect the same as a side affect?
No, these two terms do not have the same meaning. An adverse event (or experience) describes an unfavorable event or experience that occurs after a participant begins the research study. The event or experience may be reported by the research participant (such as “I was feeling dizzy all day”) or observed by the researcher (such as an abnormal lab test result). The occurrence of an unfavorable experience or event does not necessarily mean that it is associated with (or caused by) the experimental drug, device or treatment. Generally, an adverse event/experience is considered a “side effect” when, after the study is completed, the event was observed to occur much more frequently in participants who are in the experimental group than in the control group.
Questions to Ask
Anyone interested in participating in a clinical study should know as much as possible about the study and feel comfortable asking the research team questions about the study, the related procedures, and any expenses. The following questions may be helpful during such a discussion. Answers to some of these questions are provided in the informed consent document. Many of the questions are specific to clinical trials, but some also apply to observational studies.
- What is being studied?
- Why do researchers believe the intervention being tested might be effective? Why might it not be effective? Has it been tested before?
- What are the possible interventions that I might receive during the trial?
- How will it be determined which interventions I receive (for example, by chance)?
- Who will know which intervention I receive during the trial? Will I know? Will members of the research team know?
- How do the possible risks, side effects, and benefits of this trial compare with those of my current treatment?
- What will I have to do?
- What tests and procedures are involved?
- How often will I have to visit the hospital or clinic?
- Will hospitalization be required?
- How long will the study last?
- Who will pay for my participation?
- Will I be reimbursed for other expenses?
- What type of long-term follow-up care is part of this trial?
- If I benefit from the intervention, will I be allowed to continue receiving it after the trial ends?
- Will results of the study be provided to me?
- Who will oversee my medical care while I am participating in the trial?
- What are my options if I am injured during the study?

